Our program offers an intravenous ketamine intervention for individuals with treatment resistant depression. Ketamine has long been used as an anesthetic medication. Recent evidence shows that subanesthetic doses of ketamine have rapid and robust antidepressant and anti-suicidal effects, which can have potentially life-saving relief for many individuals. Ketamine appears to work by changing the levels of glutamate, the most abundant neurotransmitter in the brain, which leads to changes in how brain cells communicate with each other and resulting in changes in emotions and thinking.
Deciphering Metacognition and Treatment Response in Depression with a Novel Digital Paradigm (Auditory MMN EEG)
Study’s Overview:
The purpose of this observational study is to assess how brain function and biological signals change in response to ketamine treatment that is given to patients with treatment-resistant depression (TRD). This data will be used to develop a computational model to represent the underlying biological and physiological activities that occur in response to ketamine treatment. This model will be developed using Electroencephalography (EEG). During the EEG, the auditory mismatch negativity (MMN) task will be done, which consists of listening to a series of alternating tones.
Eligibility:
Treatment Description:
If you would like to know more information about this study, please access our Informed Consent for the Auditory MMN EEG Study.docx

Study’s Overview:
Nitrous oxide (N2O), also known as laughing gas, is an anesthetic gas that is frequently encountered in dental offices. However, it is also used in a variety of settings including labour and delivery units, as well as operating rooms. It is inexpensive and is on the World Health Organization’s list of Essential Drugs. Nitrous oxide is believed to work as an NMDA receptor antagonist, a mechanism shared with ketamine. However, unlike ketamine, it has minimal side effects. Current evidence has demonstrated that a single administration of nitrous oxide has been shown to decrease depressive symptoms, and there is ongoing research examining the effects of nitrous oxide in depression. Thus, we intend to carry out a research study to explore the clinical efficacy and functional outcomes of repeated nitrous oxide administrations.
Sustained Mood Improvement with Laughing gas Exposure: A Randomized Controlled Pilot Trial. (SMILE Trial) – Sub-Study Protocol to include Neuroimaging
Overview:
The purpose of this sub-study is to provide a better understanding of the changes in the brain’s activity in response to nitrous oxide (N2O), also known as laughing gas, in patients with Major Depressive Disorder (MDD). Brain Scans, known as Magnetic Resonance Imaging (MRI), of patients with MDD have revealed changes in the structure and function of the brain compared to that of healthy people. While many studies commonly include MRI scans alongside interventions proposed to reduce depression symptoms, the imaging performed in this sub-study will specifically allow for the identification of changes in the brain’s structure and blood flow linked to N2O intervention.
Inclusion Criteria:
Exclusion criteria:
Treatment description (ClinicalTrials.gov Identifier: NCT05528718):
If you would like to know more information about this study, please access our Informed Consent Form for the SMILE Substudy.docx
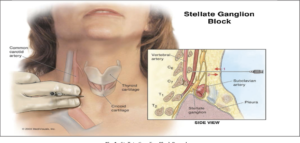
Overview:
The stellate ganglion is a part of the cervical sympathetic chain, and it provides sympathetic input to the ipsilateral upper extremity, chest, head, and neck. Using ultrasound guidance, an anesthetic block of the stellate ganglion can affect changes to neural processes which are known to be implicated in depression. Currently, an antidepressant-like effect has been demonstrated in animal models using a stellate ganglion block. This program aims to investigate whether this effect can be replicated in humans.
Eligibility:
Intervention Protocol:
This is a pilot study investigating a Stellate Ganglion Block, a new intervention for major depressive disorders. The first step of the procedure involves the infusion of a sedative agent (midazolam) to induce sleep if necessary. The second step will involve the injection of Lidocaine (a local anaesthetic) into the neck to reduce pain in the region.
The third step will involve the injection of an investigational anaesthetic medication called SENSORCAINE® (5 mg/mL) using ultrasound guidance.
This procedure will take less than 30 minutes, plus 60 minutes of recovery time.
Associated Publications: