1) Thrombosis and hemostasis
We established intravital microscopy thrombosis models and demonstrated that thrombosis occurs in mice lacking two key molecules in thrombosis: fibrinogen (Fg) and von Willebrand factor (VWF). This work significantly modified the theory of thrombosis/hemostasis (JCI 2000). I found that GPV in the GPIb-V-IX complex plays an inhibitory role in thrombin-mediated platelet activation, but a supportive role in thrombus stabilization (Blood 2001); both Fg and fibrin play roles in thrombus stabilization and plasma Fg inhibits platelet fibronectin (Fn) internalization (Blood 2003); plasma Fn promotes thrombus growth in injured arterioles (PNAS 2003). My lab further showed that Fg/VWF-independent platelet aggregation occurs in vitro under more physiological conditions (i.e. non-anticoagulated blood), and both platelet and plasma proteins contribute to this novel platelet aggregation pathway (J Thromb Haemost 2006). Interestingly, we found that soluble plasma Fn is a potent inhibitor of platelet aggregation (Blood 2009). By switching between a soluble and insoluble form, Fn plays a self-limiting regulatory role in thrombosis/hemostasis (JCI 2014). Most recently, we found that “Apolipoprotein A-IV is a novel ligand of platelet β3 integrins and an endogenous inhibitor of thrombosis” (invited revision by Nature).
2) Fetal and Neonatal Alloimmune Thrombocytopenia (FNAIT)
We established the first animal model of Fetal and Neonatal Alloimmune Thrombocytopenia (FNAIT); clarified the correlation between maternal antibody titers and pathological severity in FNAIT; and found that IVIG is a useful therapy for this life-threatening disease (Blood 2006). We further demonstrated that the neonatal Fc receptor (FcRn) plays a key role in FNAIT pathogenesis and can be targeted for therapy (Blood 2010); maternal immune response against GPIbα causes frequent miscarriage due to thrombus formation in placenta (JCI 2011, with editorial commentary and media coverage); antibody-mediated immune suppression can prevent FNAIT (Transfusion 2012, with editorial commentary; initiation of a large clinical trial in 4 European countries); impairment of angiogenesis but not thrombocytopenia is the major cause of intracranial hemorrhage in FNAIT and can be prevented with IVIG (JCI 2015). Our preliminary data suggest that maternal immune response against fetal β3 integrins (αIIbβ3 or αvβ3) on vessels, trophoblasts, and placental stem cells may play key roles in intrauterine growth restriction and miscarriage.

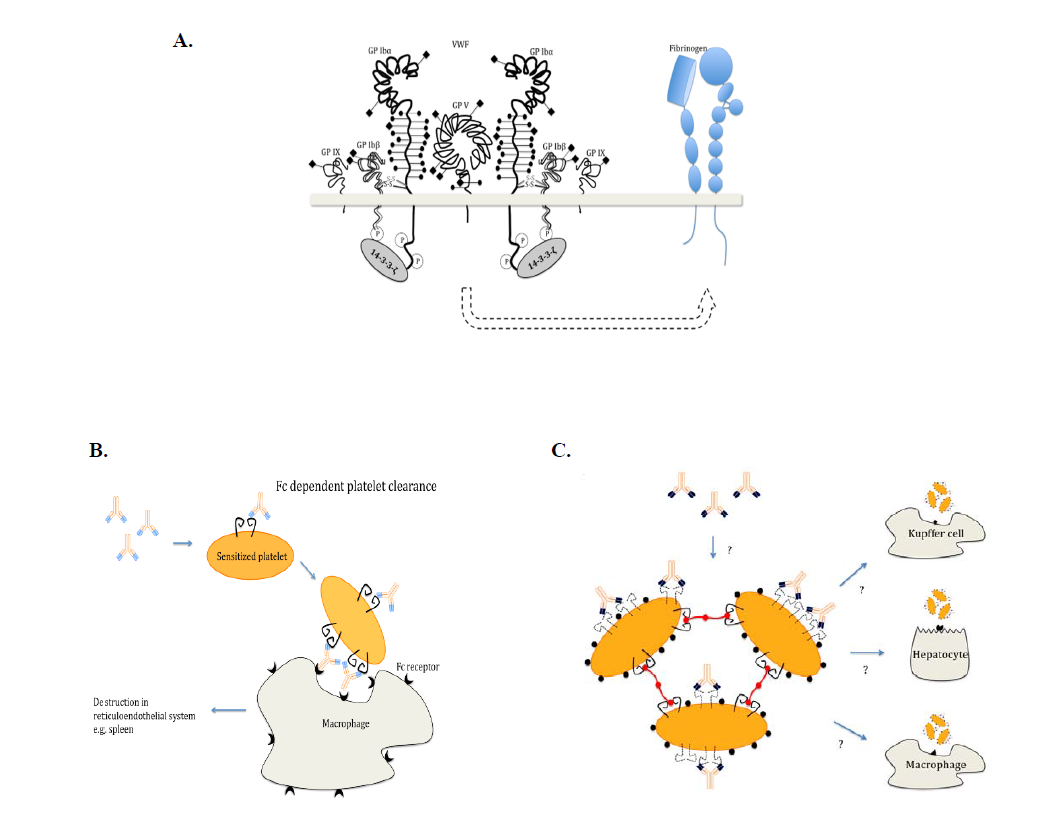
3) Immune thrombocytopenia (ITP)
We established models of immune thrombocytopenia (ITP) using our unique mouse anti-mouse αIIbβ3 and GPIbα antibodies. We found ITP induced by anti-GPIbα may fundamentally differ from that of anti-β3 integrin antibodies. The former can deliver signals to platelets and cause platelet activation/desialylation, leading to Fc-independent phagocytosis, which can be resistant to IVIG therapy. Some of these data were published (Blood 2006) and confirmed in humans by others (Haematologica. 2007) and ourselves (J Thromb Haemost 2014). Most recently, we found this Fc-independent platelet clearance occurred in liver via hepatocyte Ashwell-Morell receptors (Nature Communications 2015). This research could save Canada $10 million/year ($100 million in US and Europe/year) by identifying patients that are refractory to IVIG therapy. Two patents have been approved stemming from this project, and new sialidase inhibitor therapies have been initiated in clinical trials in China and Canada. We also identified several CD8 T + regulatory cells from this project (Blood 2015).