Acute respiratory distress syndrome (ARDS) is a life threatening condition commonly encountered in intensive care units (ICU). It can be caused by several triggers, including pneumonia or systemic sepsis and it is characterized by widespread injury of the alveolar–capillary membrane, resulting in non-cardiogenic pulmonary edema (fluid accumulation in the lungs). ARDS results in severe hypoxemia, which is refractory to oxygen treatment and requires assisted ventilation.
The annual incidence of ARDS is 13-23 people per 100,000 in the general population worldwide and in Canada only, over 10,000 people die each year of ARDS. Despite advances in ICU care, the mortality from ALI/ARDS is still very high (35-46%; Bellani et al., 2016). ARDS sufferers require mechanical ventilation to support life, which unfortunately may result in additional injury to the lungs – this is termed ventilator induced lung injury (VILI). Currently there are no pharmacological therapies for ALI/ARDS or VILI, hence treatment is entirely supportive (Laffey & Kavanagh, 2017). Understanding the molecular mechanisms of lung injury and repair is warrant and finding new treatments for ARDS is of the highest priority.
Our research is exactly focused on understanding the mechanisms underlying VILI, pneumonia induced ARDS (of endotoxic or microbial origin) and of septic injury. We are investigating the therapeutic potential of cell-based therapies for ARDS and for sepsis. We have developed several clinically relevant animal models of ARDS and sepsis and we are exploring the efficacy of mesenchymal stem/stromal cells (MSCs) from different sources (bone marrow and umbilical cord) in these preclinical models. We have found that intratracheal MSC therapy enhanced recovery in rats after VILI, and is as effective as intravenous MSC therapy (Curley et al., 2011 and 2013) MSCs derived from umbilical cords are also effective in reducing pneumonia induced lung injury (Curley et al, 2017).
Our group is investigating the mechanisms of action of MSCs, and are particularly focused on their immuno-modulatory properties (Figure 1). We have shown that human bone marrow (BM)-MSC therapy decreased E. coli induced pneumonia injury in rats (Devaney et al., 2015) and reduced lung bacterial burden, potentially via enhanced macrophage phagocytosis and by secretion of antimicrobial peptide, LL-37. We have demonstrated the therapeutic efficacy of cryopreserved umbilical cord (UC)-MSCs grown in xeno-free (i.e. without animal-derived component) conditions in rodent E. coli – induced ARDS (Curley at al., 2017), which is relevant from translational point of view, for future clinical trials. In addition, we have shown that lung structural morphology is considerably improved with UC-MSCs-treatment (Figure 2) and that cell treatment reduces lung tissue reactive oxidative species (ROS) concentrations and therefore reduces ROS-mediated tissue injury.
Our current work is focused on an engineering approach to improve MSC potency and their immuno-modulatory properties with a systematic investigation of ARDS potency measures in vitro and in animal models to develop a specific, advanced cellular therapy for ARDS and sepsis
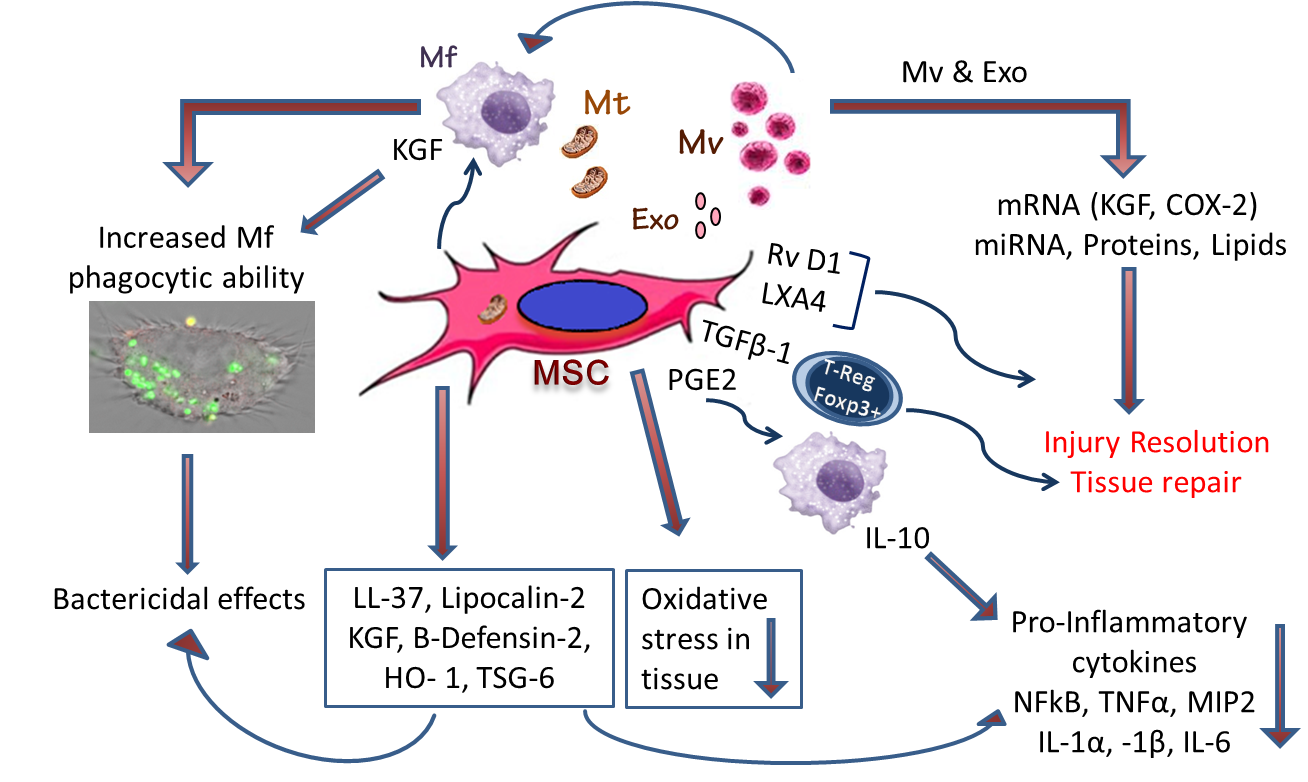
Figure 1. The pathways by which mesenchymal stem (stromal) cells (MSCs) can reduce lung and multi-organ injury in sepsis, increase phagocytic abilities of macrophages and facilitate resolution of injury:
Mechanistic insights, elucidating the role of MSC secreted paracrine effectors, including keratinocyte growth factor, HO-1 and TSG-6 in modulating the immune response (reducing NFkB, TNF-alpha and MIP-2, increasing IL-10 concentrations), and in promoting injury resolution and repair. The MSC secretome and MSC-derived microvesicles also effectively attenuate tissue injury.
Mitochondrial transfer from MSCs to the epithelium appears important in reducing the severity of injury. MSCs promote repair by releasing factors as Resolvin D4, Lipoxin A4 (LXA4), and TGFβ1, which increases regulatory Foxp3+ T-cell population, known for their integral role in epithelial regeneration. They also reduce neutrophil influx and their activation, and enhance resolution of inflammation by the increased release of anti-inflammatory cytokine interleukin-10 (IL-10), and decreased release of pro-inflammatory molecules, mechanisms that are mediated by LXA4 and prostaglandin E2 (PGE2). MSCs also equip macrophages (Mɸ) with machinery that increases their ability to phagocytose and kill bacteria, processes mediated by release of the antimicrobial peptide, beta-cathelicidin (LL-37), KGF, and HO-1 and by the transfer of microvesicles and mitochondria to macrophages from MSCs.
Abbreviations: MSC, mesenchymal stem/stromal cell; Mf, macrophages; Mv, macrovesicles; Exo, exosomes; Mt, mitochondria; KGF, keratinocyte growth factor; TNF, tumor necrosis factor; MIP2, macrophage inflammatory protein 2; RvD1, resolving D1; LXA4, lipoxin A4; TGFβ-1, transforming growth factor β-1; T-Reg, Regulatory Foxp3+ T-cells; PGE2, prostaglandin E2; HO-1, Heme oxygenase-1; TNF-stimulated gene-6.
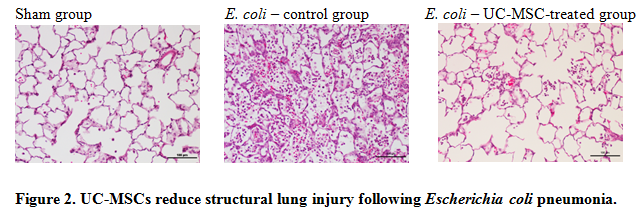
Representative photomicrographs of lung from a sham animal, a vehicle (PBS)-treated control animal, and a UC-MSC treated animal demonstrate reduced lung injury in the UC-MSC–treated group.